UCSF Researchers Identify Unique Genetic Alterations in Brain Tumors Occurring After Radiation
For many common childhood cancers, radiation therapy can be an effective, even curative, treatment. However, long-term survivors are at risk for developing new, or secondary, cancers as a consequence of radiation, sometimes decades later.
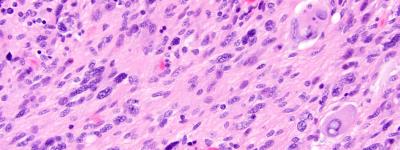
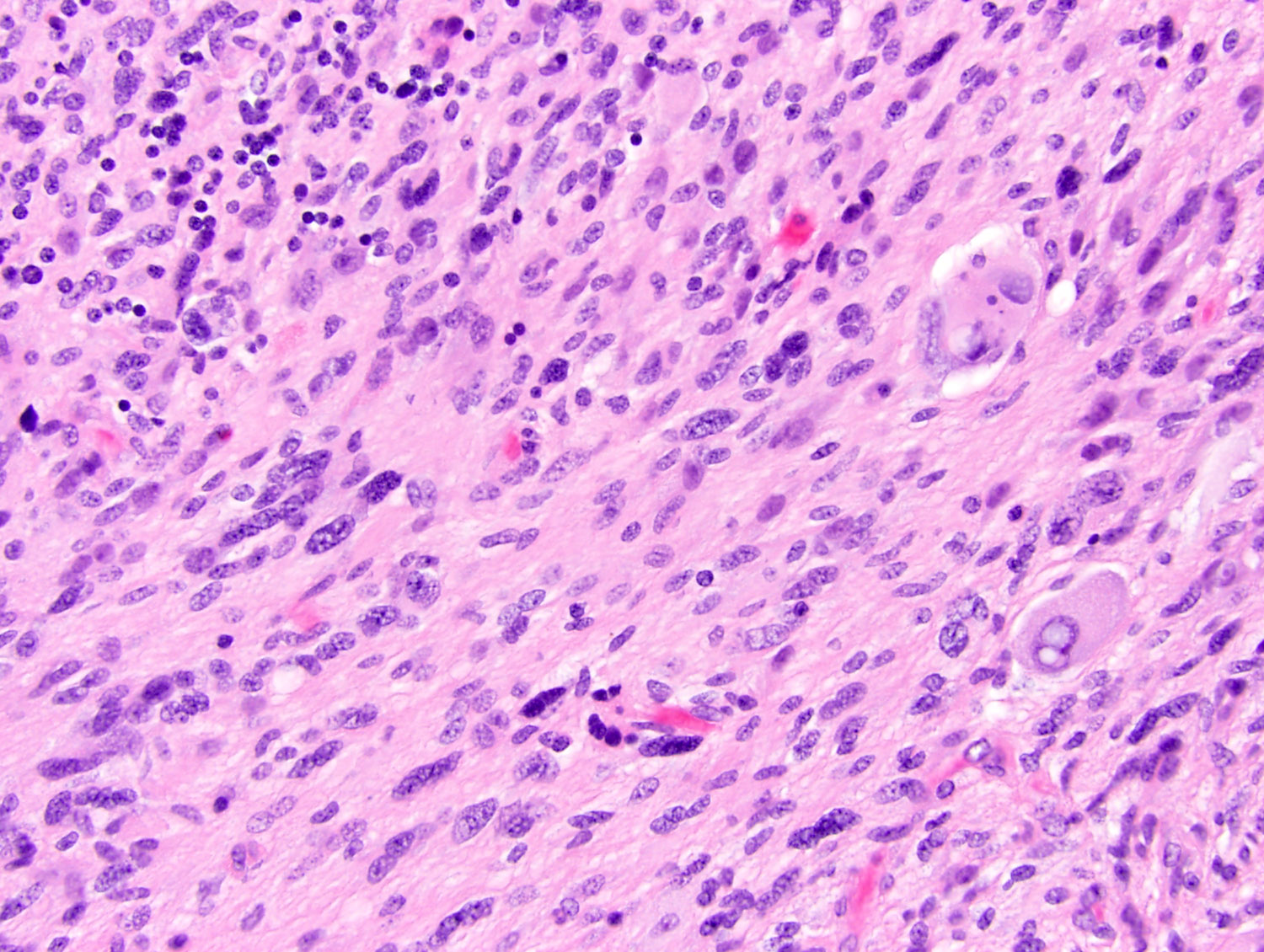
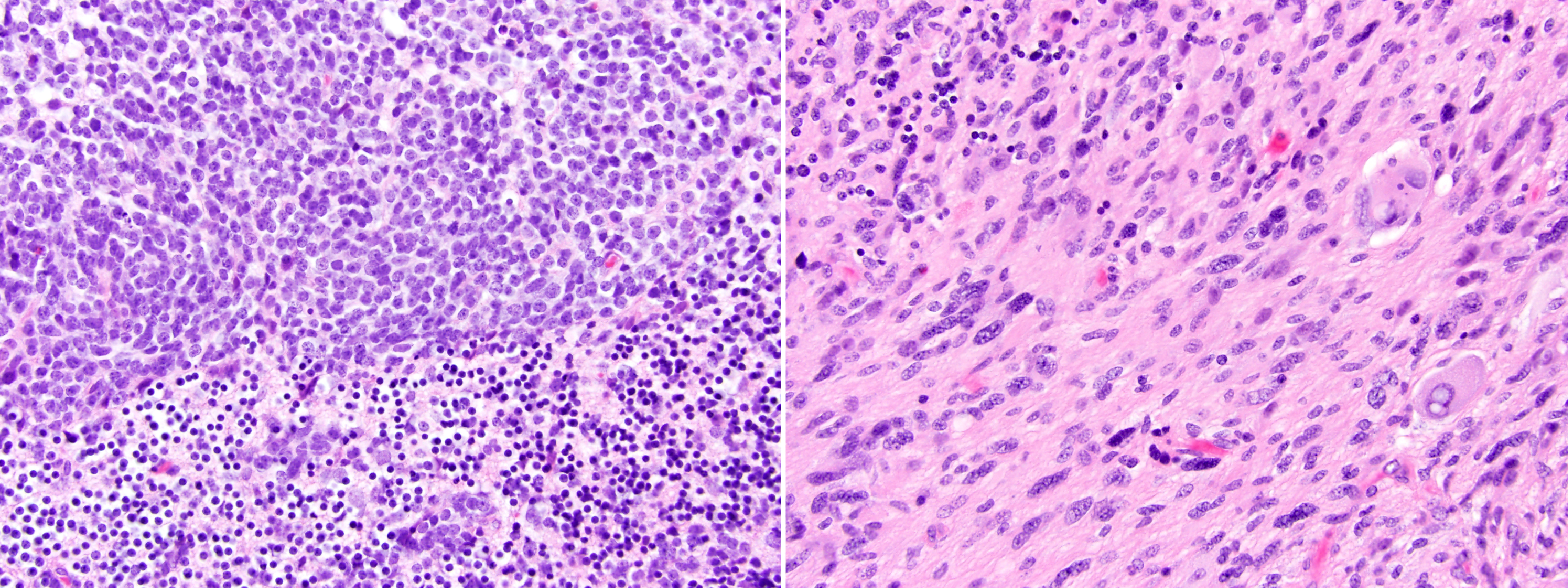
Children with cancers like medulloblastoma and craniopharyngioma are likely to receive intracranial radiation therapy as part of their treatment. A minority of these patients will later develop a secondary brain tumor as a result of radiation; these are often meningiomas or high-grade gliomas.
“Hopefully one day we’ll have more specific targeted medications to treat and cure cancer, allowing the avoidance of radiation therapy altogether. But for now, radiation is still a mainstay in the treatment regimen for many cancers,” remarked UCSF neuropathologist David Solomon, MD, PhD.
Secondary cancers represent a major cause of mortality for long-term survivors of childhood cancers, and much remains unknown about how these tumors arise. Recently published in Acta Neuropathologica, Solomon led a team of UCSF clinicians and researchers in the first genomics study of radiation-induced gliomas.
Solomon and his colleagues found that radiation-induced gliomas carry a unique genetic signature that is distinguishable from that of spontaneous gliomas. Radiation, by causing double stranded breaks in DNA, leaves a distinct pattern of chromosomal breakpoints that result from the faulty repair of DNA damage.
They further identified which genes are commonly mutated in radiation-induced gliomas, which are different from those commonly found in spontaneous gliomas.
Many pediatric and adult gliomas are defined by IDH1, IDH2, TERT promoter, PTEN, or H3 K27M mutations, depending on the exact subtype. However, radiation-induced gliomas lack mutations in those genes, instead carrying mutations in genes like CDK4, TP53, and PDGFRA, which represent disruptions to cell cycle regulation, tumor suppressor genes, and other signaling pathways implicated in tumor growth.
“These findings demonstrate the importance of molecular sequencing of brain tumors, both from a diagnostic and therapeutic standpoint,” explained UCSF pediatric neuro-oncologist Cassie Kline, MD, who is a co-author on the paper.
At UCSF, an increasing number of patients with glioma undergo DNA sequencing of their tumor, although this is not as common elsewhere. The UCSF500 Cancer Gene Panel test identifies mutations that are present in the tumor sample, allowing clinicians to determine which targeted therapies would be more effective.
By identifying the genetic alterations common to radiation-induced gliomas, Solomon and his team were able to find several mutations that could be potentially targeted with existing FDA-approved small molecule inhibitors.
With effective targeted therapies, radiation-induced gliomas could be treated with targeted drugs – rather than more radiation – that have less overall toxicity and fewer side effects or long-term consequences. “The more we know, the better we can find a tailored treatment,” said Solomon.
“Eventually,” he predicts, “we’ll be able to treat cancers based on their exact genetic mutations and match the most appropriate therapies.”
Read the Solomon Lab's latest publication to learn more about the genetics of radiation-induced glioma.